Background:
Myelofibrosis (MF) is a BCR-ABL1-negative myeloproliferative neoplasm (MPN) associated with JAK2 V617F mutation in approximately 50% of patients. Allogeneic stem cell transplantation (alloSCT) is the only curative treatment option for MF but a significant proportion of patients undergoing alloSCT experience relapse. Although measurable residual disease (MRD) is an established predictor of relapse in other hematological malignancies, the dynamics of JAK2 V617F variant allele frequency (VAF) in MF post-alloSCT and its prognostic impact are yet to be clearly delineated. In this study, we assessed the utility of sensitive molecular detection of JAK2 V617F as a marker of post-alloSCT outcomes in MF particularly with respect to the optimal threshold and time points for predicting early relapse.
Methods:
A retrospective analysis was conducted of consecutive patients with JAK2 V617F-mutated MF receiving alloSCT at our institution between 2016 and 2022. Data on clinicopathological features, alloSCT characteristics, treatment response assessments, graft-versus-host disease (GVHD) and outcomes were collected. JAK2 V617F was enumerated by droplet digital polymerase chain reaction (ddPCR) using the Bio-Rad QX200 ddPCR platform on archived bone marrow or peripheral blood samples at defined time points pre- and post-alloSCT. JAK2 V617F VAF was defined as the percentage of JAK2 V617F mutant copies amongst total JAK2 (mutant plus wild-type) copies, with a routine assay sensitivity of 0.05%.
Results:
31 patients with JAK2 V617F-mutated MF underwent alloSCT during the study period (Table 1). Five patients experienced relapse at a median of 14.2 (range: 3.2-22) months post-alloSCT. 13 patients died of infection (n = 5), overt relapse (n = 4) or GVHD (n = 4). After a median duration of follow-up of 17.5 (range: 0.9-77.9) months, the 5-year overall survival (OS) and relapse-free survival (RFS) were 52.8% and 49.6%, respectively. The 2-year cumulative incidences of relapse (CIR) and non-relapse mortality (NRM) were 26.6% and 35.5%, respectively. On univariate analysis, superior OS and RFS were only significantly associated with chronic GVHD ( P = 0.017 and 0.013, respectively).
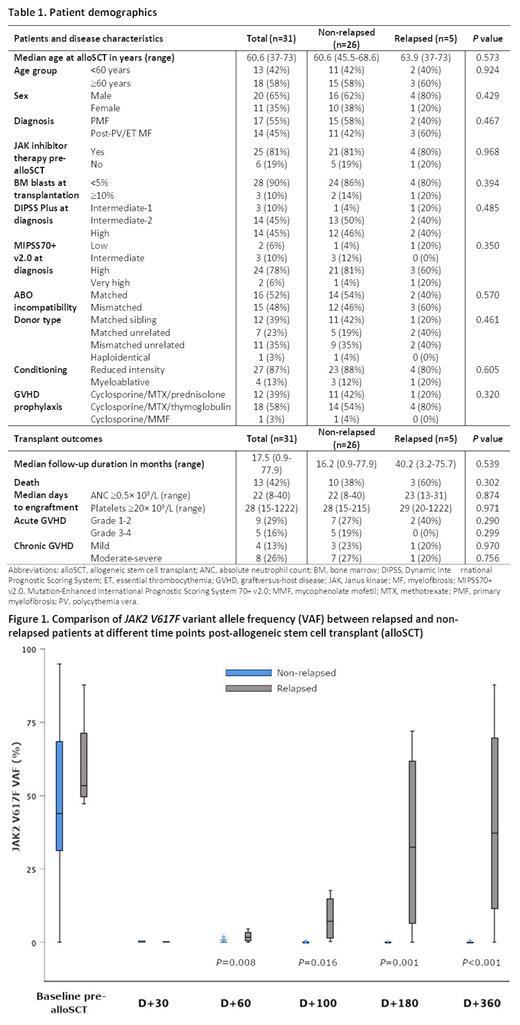
A total of 174 samples were obtained within 1 month of alloSCT (n = 31) and on days +30 (n = 31), +60 (n = 30), +100 (n = 29), +180 (n = 23) and +360 (n = 21) after alloSCT. All patients had detectable JAK2 at the time of alloSCT with a median VAF of 43.8% (range: 0.2-95%). The incidence of JA K2 V617F detection was 45.2% on day +30, 43.3% on day +60, 44.8% on day +100, 34.8% on day +180 and 23.8% on day +360 post-alloSCT. Of 6 patients who received ruxolitinib for GVHD treatment post-alloSCT, one eventually relapsed while the other 5 had undetectable JAK2 prior to ruxolitinib commencement. JAK2 VAF was higher in patients who eventually relapsed than in non-relapsed patients on days +60, +100, +180 and +360 ( P = 0.008, 0.016, 0.001 and <0.001, respectively) (Figure 1). However, JAK2 positivity at each of these time points was not significantly associated with survival outcomes (OS, RFS, NRM or GVHD-free relapse-free survival [GRFS]).
Receiver operating characteristic (ROC) analysis indicated that JAK2 VAF on days +100, +180 and +360 post-alloSCT were significant predictors of eventual relapse ( P = 0.022, 0.019 and 0.01, respectively). The optimal JAK2 VAF thresholds at each of these time points were 0.2% (sensitivity 80%; specificity 83.3%; area under curve [AUC] 0.788) at day +100, 7.1% (sensitivity 75%; specificity 94.7%; AUC 0.842) at day +180, and 11.7% (sensitivity 75%; specificity 94.1%; AUC 0.86) at day +360. Comparisons of JAK2 VAF at each time point to the immediately preceding VAF showed that ≥2-fold increases at days +100 (from day +60) and +180 (from day +100) were significantly associated with relapse ( P = 0.002 and 0.001 respectively).
Conclusion:
Although persistence of JAK2 is common for more than a year post-alloSCT for JAK2 V617F-mutated MF, serial sensitive molecular monitoring of JAK2 at defined time points appears to be a predictive marker of relapse. In this study, doubling of JAK2 VAF at days +100 and +180 post-alloSCT were associated with relapse. These data support the notion that sensitive molecular detection of JAK2 may have a role akin to MRD in informing the pre-emptive use of immunotherapeutic intervention in this setting, including immunosuppression withdrawal and/or donor lymphocyte infusions.
Disclosures
Szer:Alexion, AstraZeneca Rare Disease: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Prevail Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Apellis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Ritchie:BMS: Research Funding; Amgen: Research Funding; MSD: Honoraria; Novartis: Honoraria, Research Funding; Takeda: Consultancy, Honoraria. Chee:Keros Therapeutics: Honoraria; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Otsuka: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal